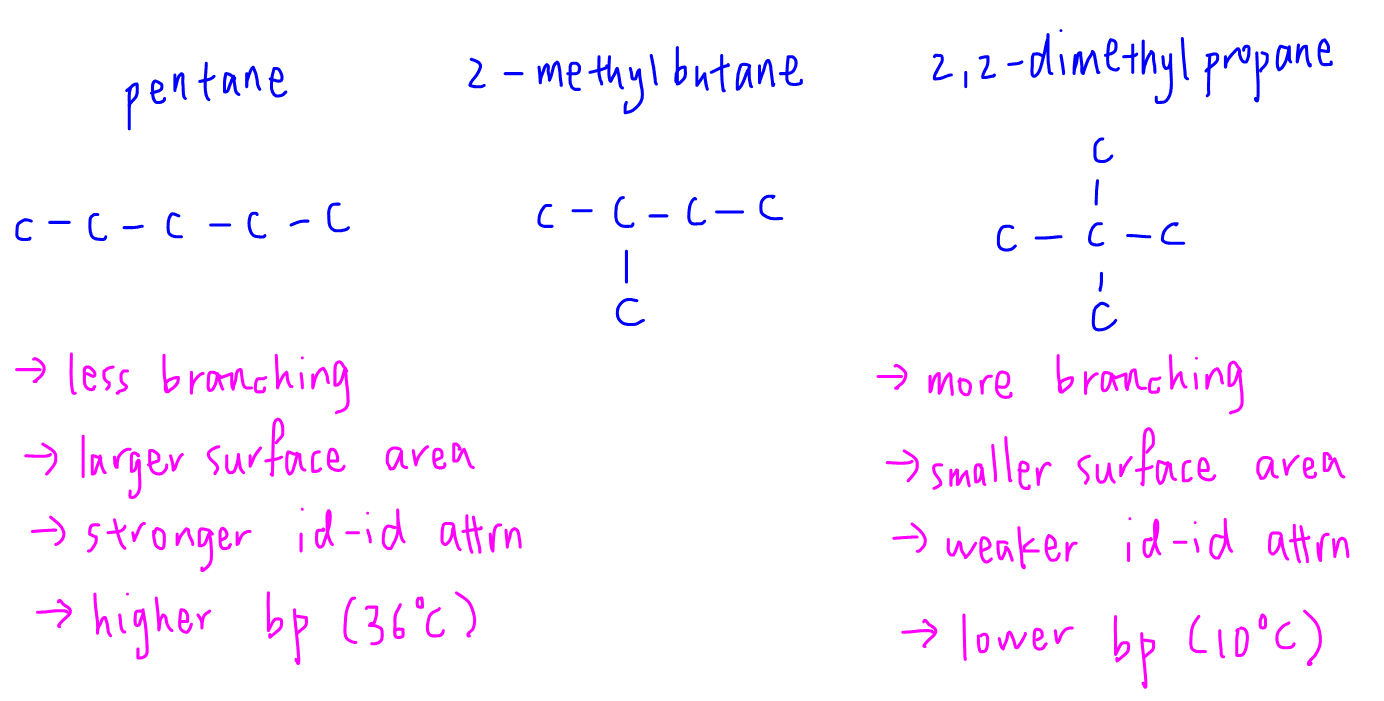CHEM 3102 Sapling Week 8: Exp 3.3 A&B: Structural effects of boiling point and refractive Index & Unknown Liquid Flashcards | Quizlet

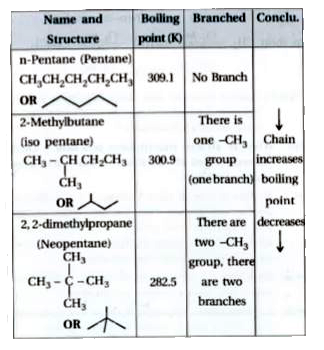
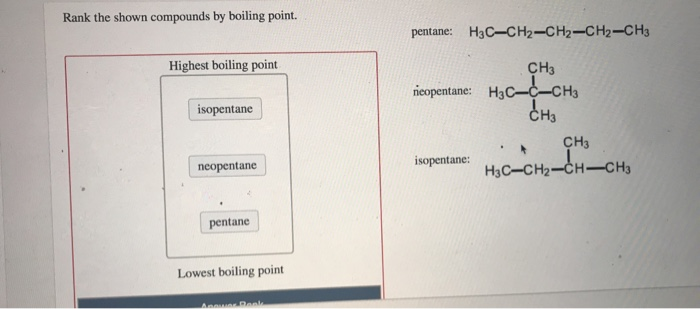
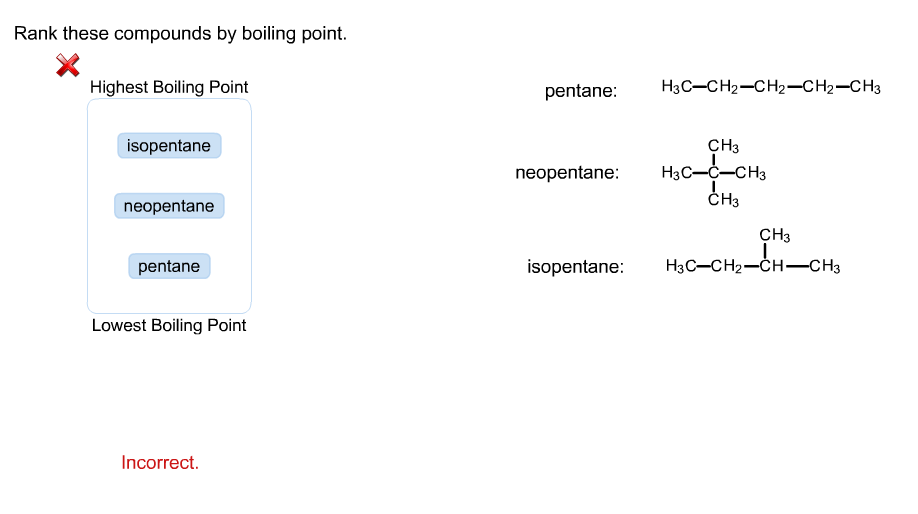
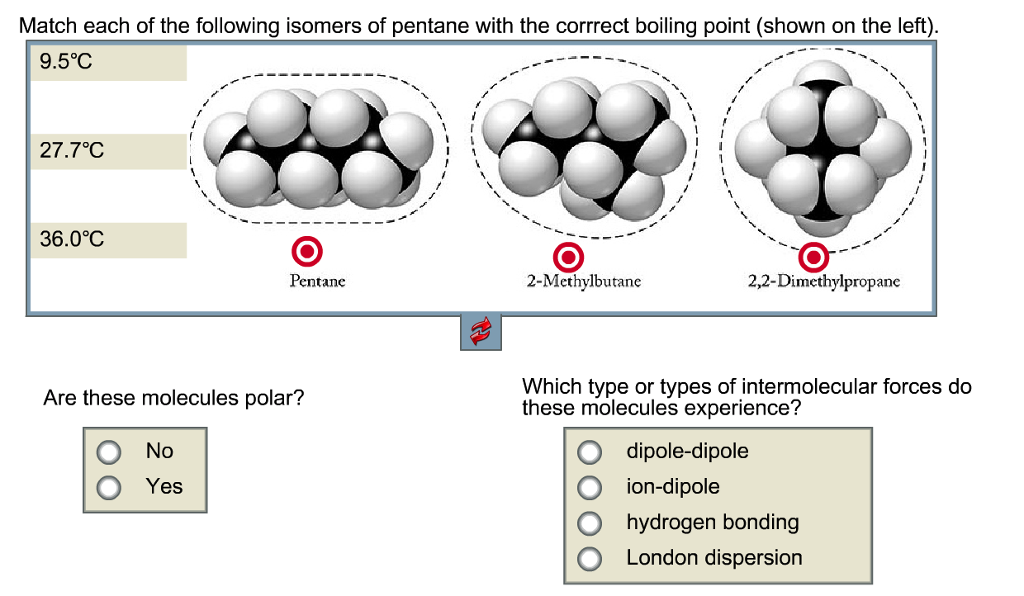
Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane

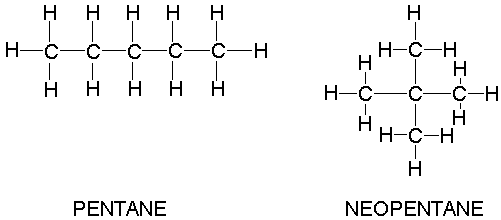
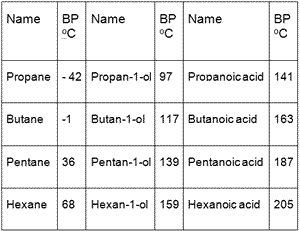
Pentane has a boiling point of 36.1 degrees Celsius while 1-butanol, which has a similar mass, has a boiling point of 117.7 degrees Celsius. Explain this difference, including line-angle structures of each
21. Why The boiling point of pentane is greater than isopentane? And why the boiling point of neopentane is less than N pentane and isopentane?

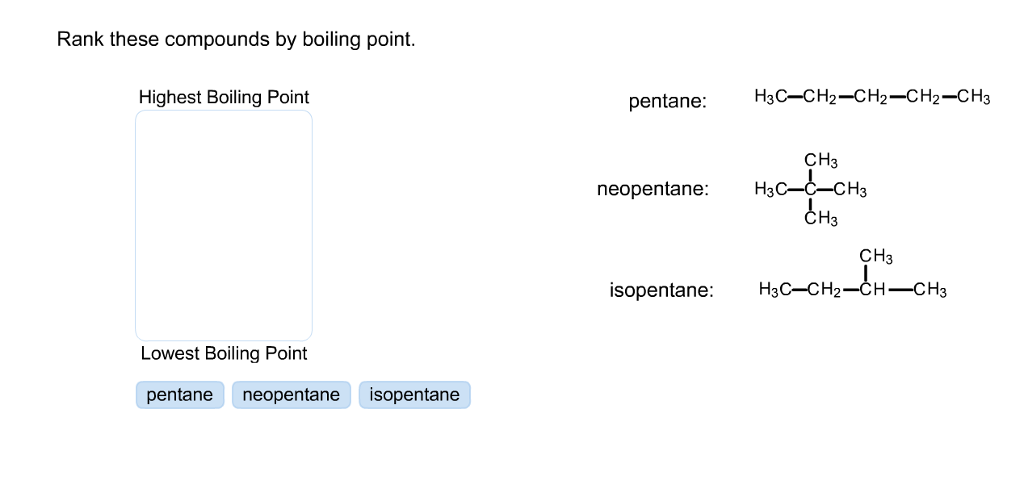
Rank these compounds from highest to lowest boiling point. a. pentane b. neopentane c. isopentane | Homework.Study.com
Why Isomers of a compound have different Boiling point (like Isomers of pentane) why force of attraction is not involve in it? - Quora

Which of the following has the highest boiling point ? i. 2-Methyl pentane ii. 2,3-Dimethyl - YouTube

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane
![SOLVED: Arrange the compounds by boiling point: pentane: HjCCHz-CHz-CHz-CH] Highest boiling point CHy neopentane: HjCC-CHj CH3 hexane: H;CCHz-CHz CHz-CHz CH, Lowest boiling point Answer Bank neopentane pentane hexane SOLVED: Arrange the compounds by boiling point: pentane: HjCCHz-CHz-CHz-CH] Highest boiling point CHy neopentane: HjCC-CHj CH3 hexane: H;CCHz-CHz CHz-CHz CH, Lowest boiling point Answer Bank neopentane pentane hexane](https://cdn.numerade.com/ask_images/e17b5f312e434896a1aa14c6e26bbf4f.jpg)
SOLVED: Arrange the compounds by boiling point: pentane: HjCCHz-CHz-CHz-CH] Highest boiling point CHy neopentane: HjCC-CHj CH3 hexane: H;CCHz-CHz CHz-CHz CH, Lowest boiling point Answer Bank neopentane pentane hexane

organic chemistry - Why does neopentane have a higher melting point than n- pentane? - Chemistry Stack Exchange