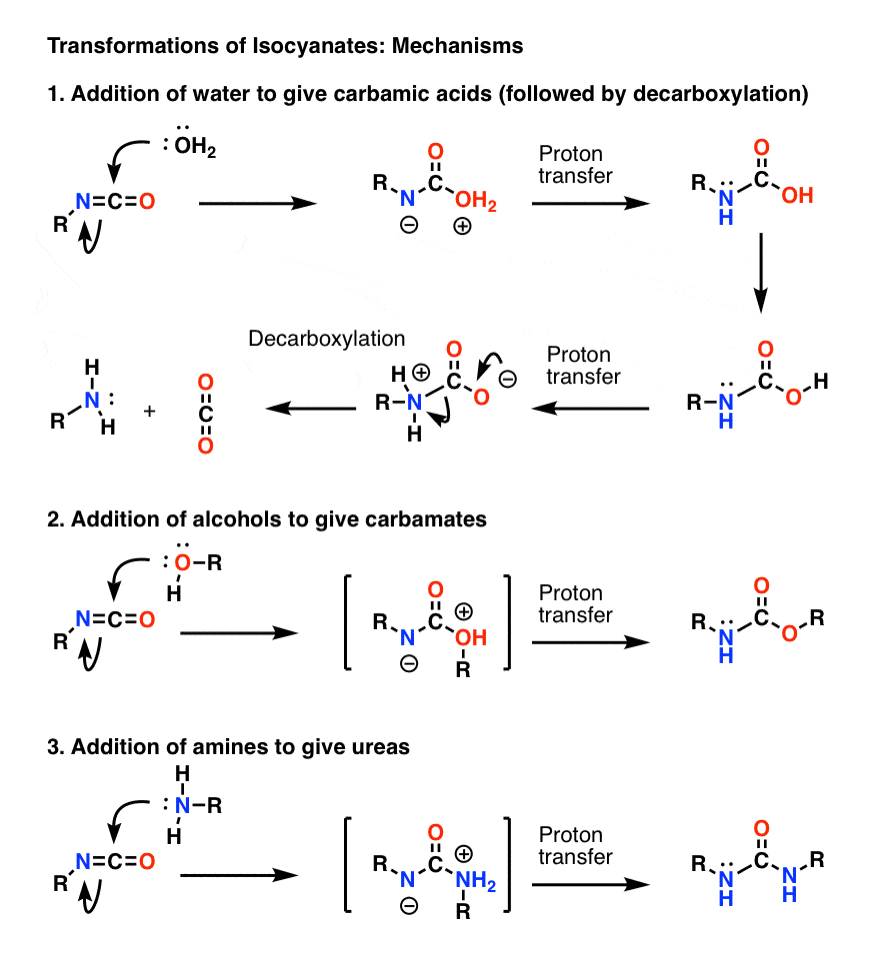
Figure 2 from Mechanism of action of organophosphorus and carbamate insecticides. | Semantic Scholar
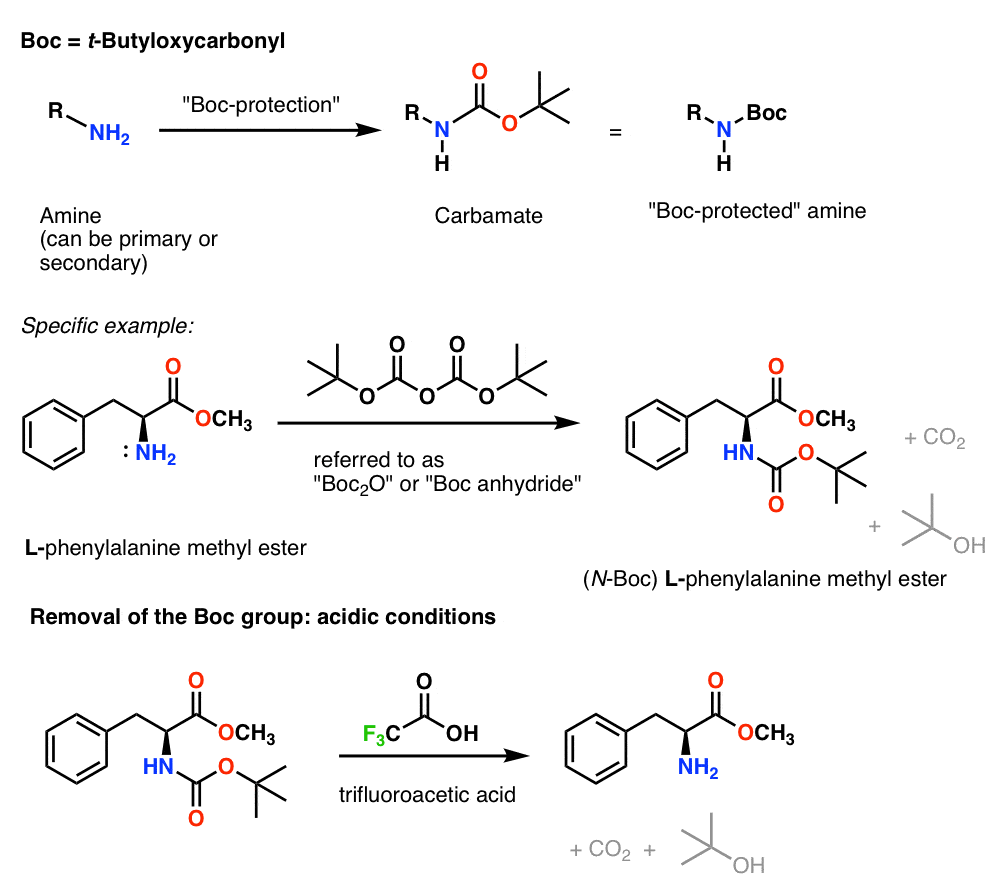
Reactivity of N -pyridylcarbamates in basic media - Journal of the Chemical Society, Perkin Transactions 2 (RSC Publishing) DOI:10.1039/B200445N

SOLVED: Question 3 (25 points): The carbamate containing compound (3) , releases the free amine rapidly under basic conditions: In contrast; a carbamate is extremely stable to base hydrolysis Describe an arrow

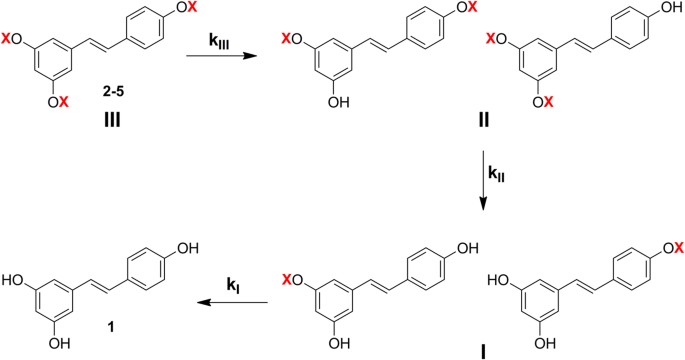
Hammett plot for hydrolysis of N -aryl pyridylcarbamates showing the e... | Download Scientific Diagram

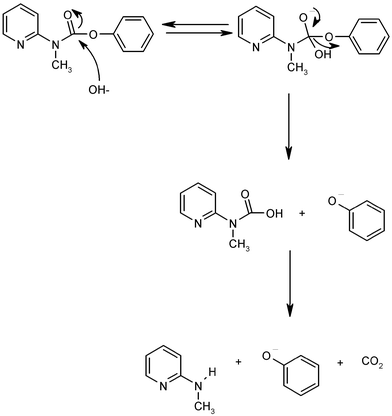
Alkaline hydrolysis of tertiary N-(2-pyridyl)carbamates. Contradictory evidence between nucleophilic and general base catalysis | SpringerLink
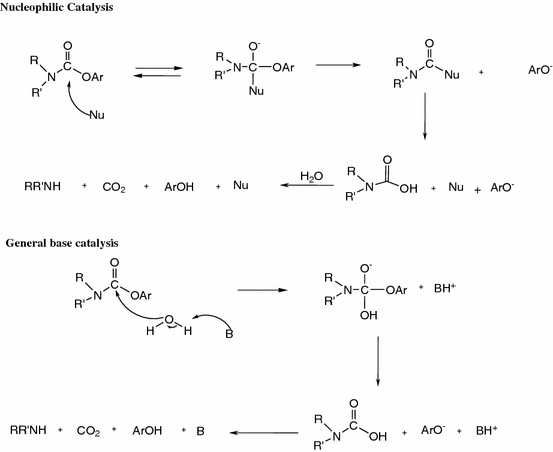
Alkaline hydrolysis of tertiary N-(2-pyridyl)carbamates. Contradictory evidence between nucleophilic and general base catalysis | SpringerLink

Hydrolysis is the most commonly encountered drug degradation mechanism, both in solution and in the solid state. Use the structure of ethyl ethanoate below to illustrate the mechanism of acid-catalyzed hydrolysis of

The identification of carbon dioxide mediated protein post-translational modifications | Nature Communications

IJMS | Free Full-Text | Hydrolysis Mechanism of Carbamate Methomyl by a Novel Esterase PestE: A QM/MM Approach
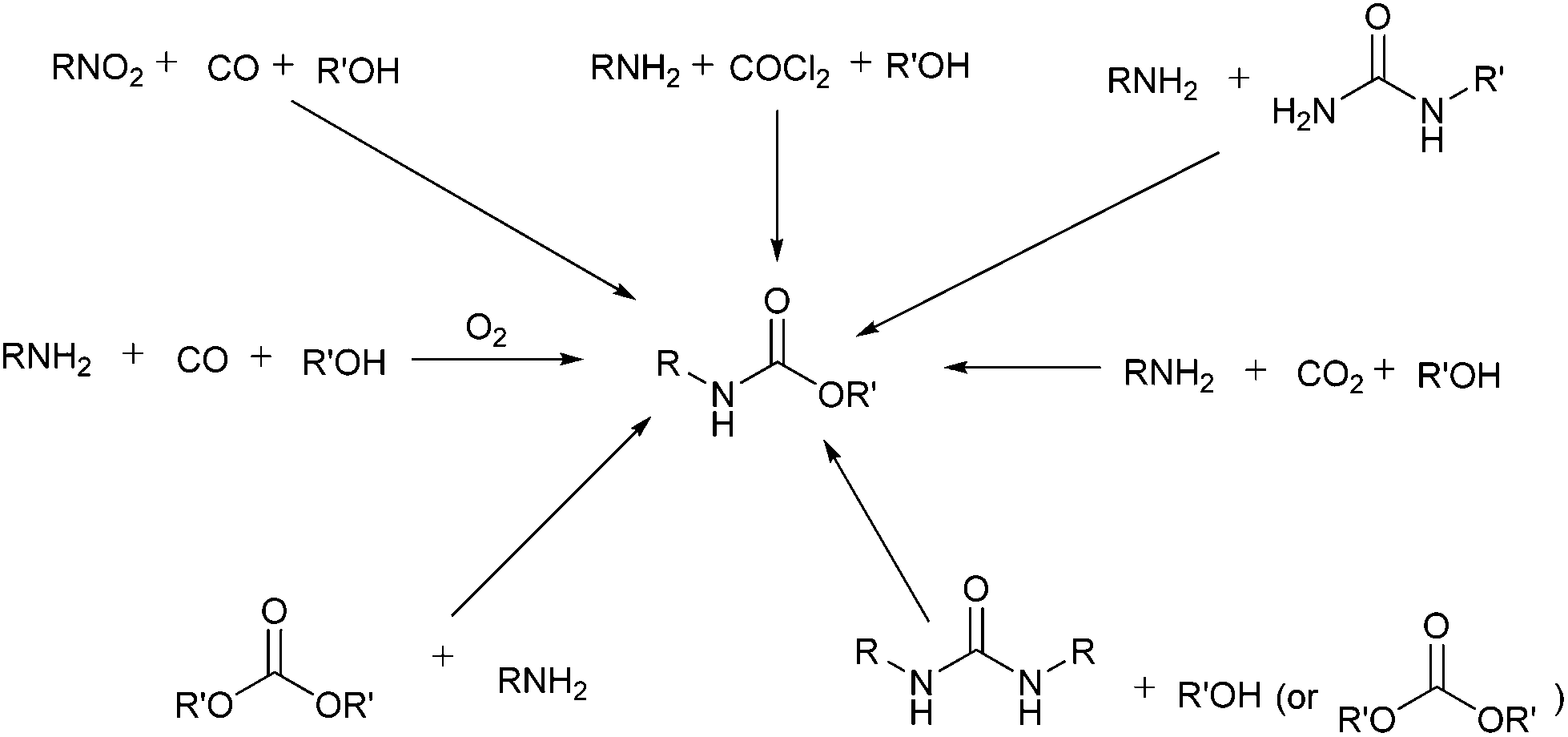
N -Substituted carbamate synthesis using urea as carbonyl source over TiO 2 –Cr 2 O 3 /SiO 2 catalyst - Green Chemistry (RSC Publishing) DOI:10.1039/C5GC01007A
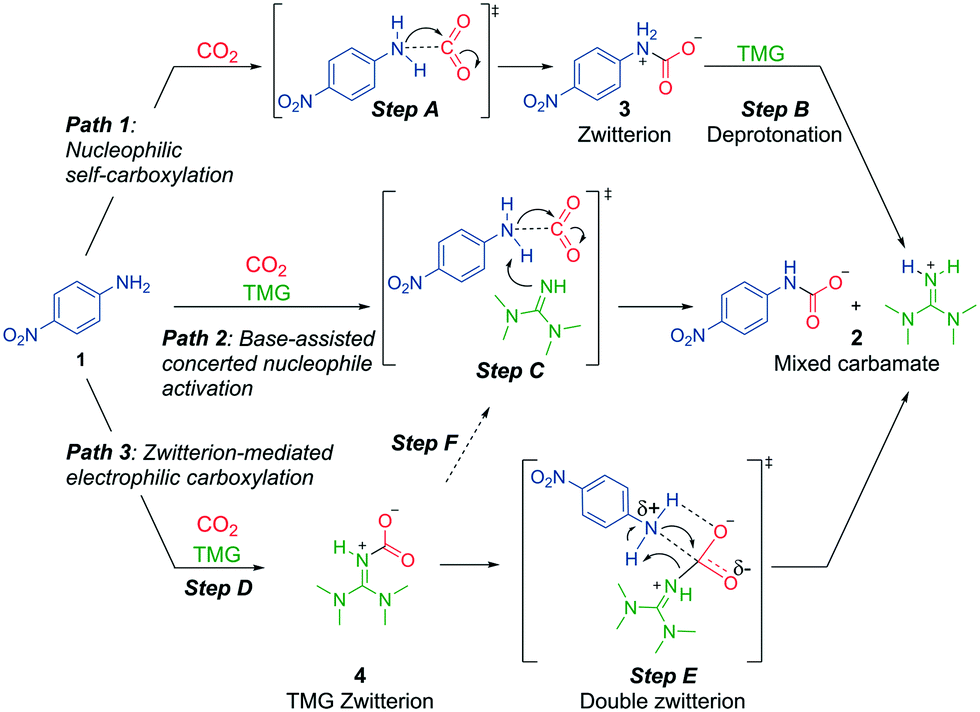
Mechanistic insights into carbamate formation from CO 2 and amines: the role of guanidine–CO 2 adducts - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/D1CY01433A

SciELO - Brasil - Kinetics and mechanism of hydrolysis of benzimidazolylcarbamates Kinetics and mechanism of hydrolysis of benzimidazolylcarbamates
Carbamate group as structural motif in drugs: a review of carbamate derivatives used as therapeutic agents