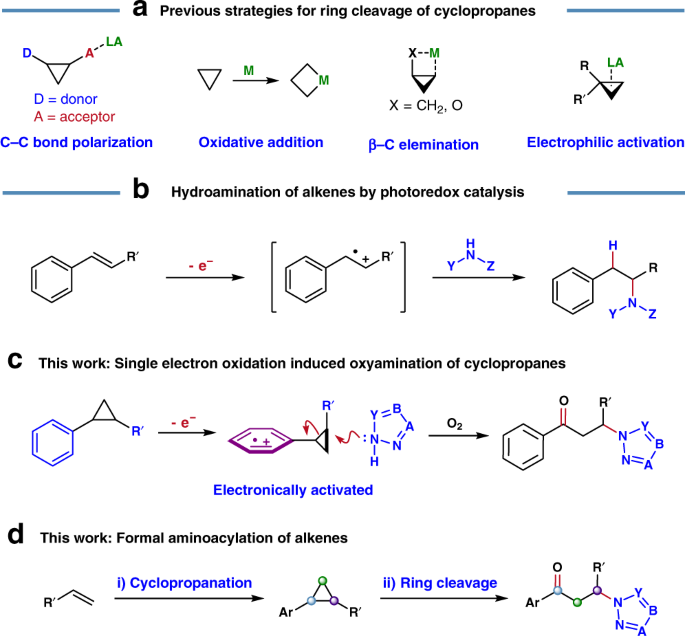
Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol - Chemical Science (RSC Publishing) DOI:10.1039/C8SC02126K

Visible‐Light‐Mediated Ring‐Opening Reactions of Cyclopropanes - Sivanandan - 2021 - European Journal of Organic Chemistry - Wiley Online Library
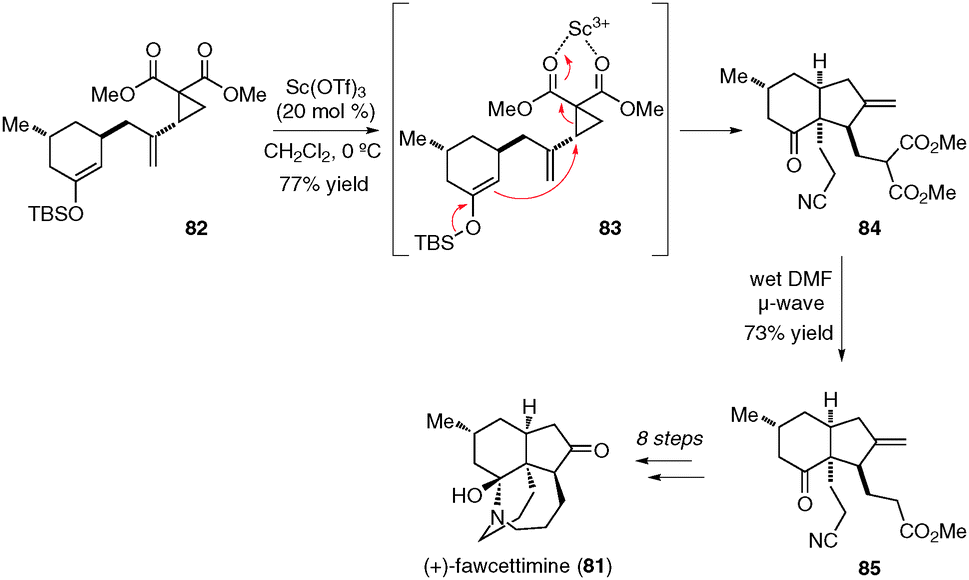
Intramolecular donor–acceptor cyclopropane ring-opening cyclizations - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60238A
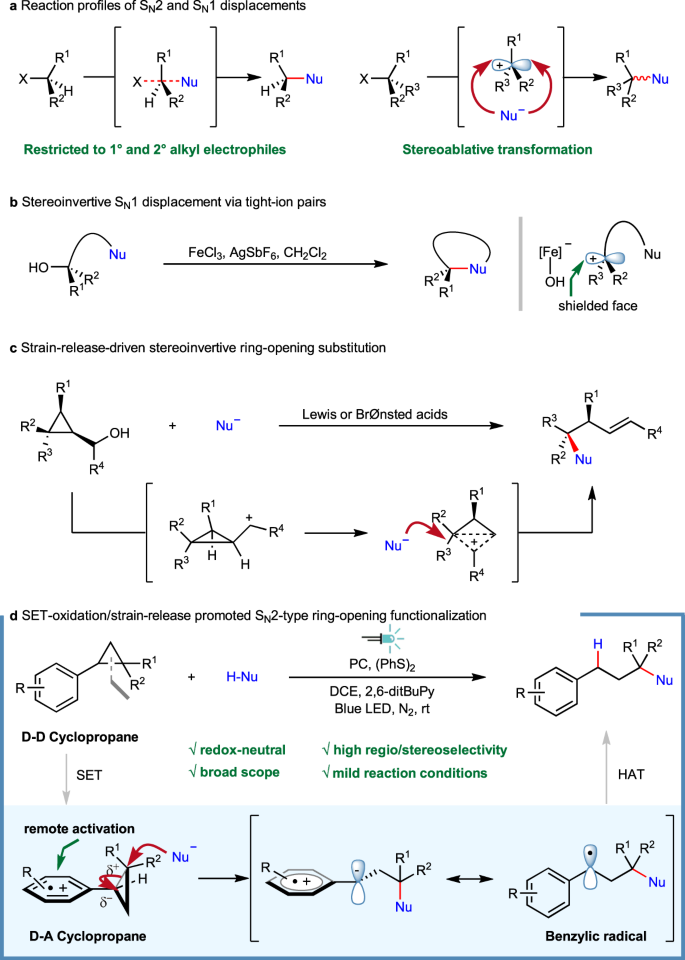
Photoredox-catalyzed C–C bond cleavage of cyclopropanes for the formation of C(sp3)–heteroatom bonds | Nature Communications

Cyclopropylcarbinyl-Type Ring Openings. Reconciling the Chemistry of Neutral Radicals and Radical Anions | Journal of the American Chemical Society

Ring opening of cyclopropylcarbinyl radicals resulting from hydrogen... | Download Scientific Diagram

A Vinyl Cyclopropane Ring Expansion and Iridium‐Catalyzed Hydrogen Borrowing Cascade - Wübbolt - 2020 - Angewandte Chemie - Wiley Online Library

Stereoelectronic and Resonance Effects on the Rate of Ring Opening of N- Cyclopropyl-Based Single Electron Transfer Probes | Journal of the American Chemical Society

organic chemistry - How do I decide whether ring will expand in this reaction or not? - Chemistry Stack Exchange

Ring-Opening Dynamics of the Cyclopropyl Radical and Cation: the Transition State Nature of the Cyclopropyl Cation | Journal of the American Chemical Society
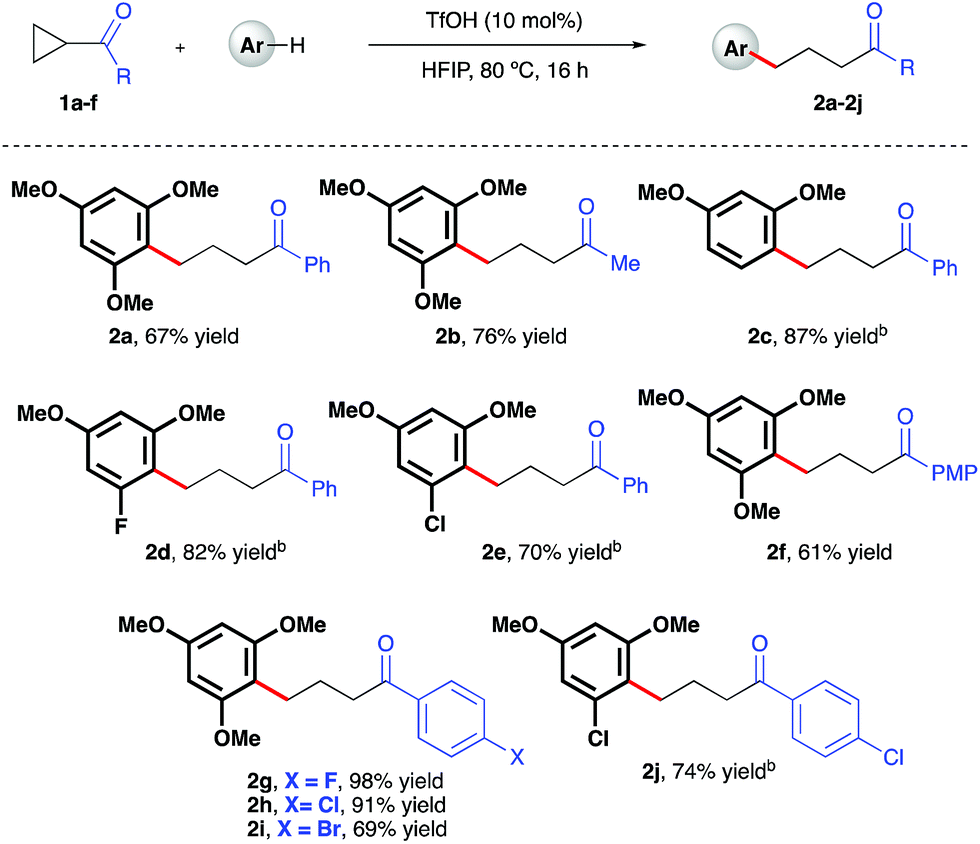
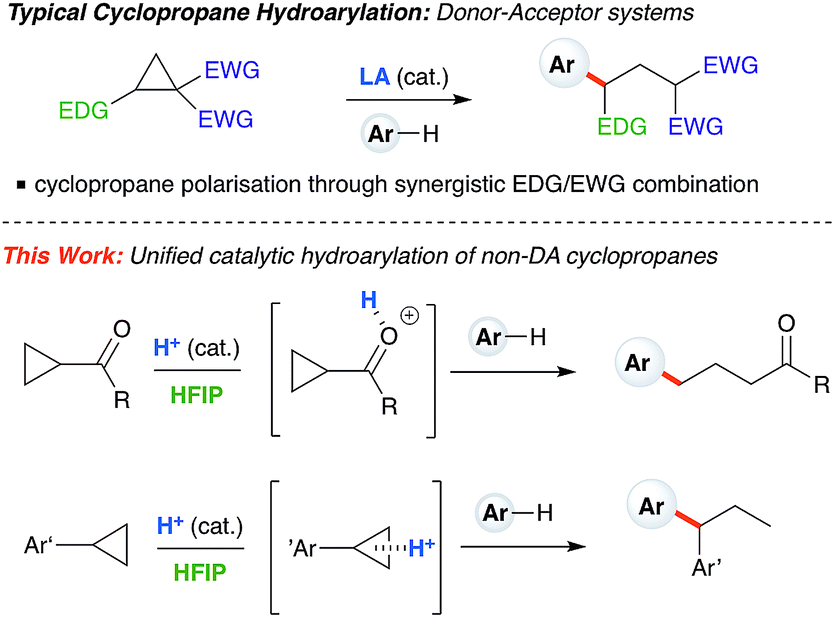
Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol - Chemical Science (RSC Publishing) DOI:10.1039/C8SC02126K

Stereoelectronic and Resonance Effects on the Rate of Ring Opening of N- Cyclopropyl-Based Single Electron Transfer Probes | Journal of the American Chemical Society

Figure 1 from Enantioselective palladium(0)-catalyzed intramolecular cyclopropane functionalization: access to dihydroquinolones, dihydroisoquinolones and the BMS-791325 ring system† †Electronic supplementary information (ESI) available: Experimental ...